Achondroplasia
软骨发育不全是一种罕见的遗传性骨骼生长状况,其特征是不成比例的身材矮小, curvature of the spine and an enlarged head (macrocephaly).
临床试验评估研究药物的安全性和有效性,并需要支持新药的监管批准. After a medicine is approved, 临床试验在帮助进一步了解治疗效果方面也发挥着重要作用. 观察性研究是另一种类型的临床研究,研究人员通过这种研究收集数据并评估健康结果,而不改变参与者的护理.
了解更多关于博彩平台网址大全正在进行的临床试验. 如果您有兴趣了解有关特定试验的更多信息,请发送电子邮件 medinfo@bmrn.com.
软骨发育不全是一种罕见的遗传性骨骼生长状况,其特征是不成比例的身材矮小, curvature of the spine and an enlarged head (macrocephaly).
CLN2(晚期婴儿神经性脑蜡样脂肪褐变2型)疾病是一种超罕见且进展迅速的儿童脑部疾病,也是神经性脑蜡样脂肪褐变最常见的形式之一, 这是一组遗传性疾病,统称为巴顿病.
Duchenne muscular dystrophy (DMD) is a recessive, 由严重缺乏或完全缺乏肌营养不良蛋白引起的x连锁神经肌肉疾病, a protein that helps protect muscle cells.
A型血友病是一种罕见的出血性疾病,由提供制造FVIII蛋白指令的基因突变引起, which is essential for blood to clot normally.
HAE是一种罕见的遗传性疾病,其特征是自发肿胀,阻塞气道和阻止呼吸可危及生命. HAE的主要类型是由SERPING1基因突变引起的. 这种基因编码一种叫做C1-INH的蛋白质,这种蛋白质在控制体内某些类型的肿胀中起着重要作用. 当C1-INH缺失或不能正常工作时,可能会发生突然的、意外的肿胀事件.
软骨发育不全是一种罕见的遗传病,通常以身材矮小为特征, stocky build, disproportionately short arms and legs, broad, short hands and feet, mild joint laxity, scoliosis, and macrocephaly.
MPS IVA, also known as Morquio A, 是一种影响身体主要器官系统的罕见遗传性疾病吗. 这种情况是粘多糖病的一种形式,是一种溶酶体储存障碍.
Mucopolysaccharidosis VI (MPS VI), also known as Maroteaux-Lamy Syndrome, 遗传性溶酶体贮积症是由于缺乏分解某些复杂碳水化合物所需的酶引起的吗.
临床试验是为了评估新的潜在治疗方法而进行的一项研究. During a clinical trial, 收集信息以确定候选产品是否安全有效, as well as to evaluate the risks and benefits of the medicine. For more information about clinical trials, please visit ClinicalTrials.gov or 临床研究参与信息与研究中心
在人体临床试验开始之前,研究和开发需要经过几个阶段. 临床前测试是对每一个研究产品进行安全性评估, efficacy, best practices for administration, 在进入人体临床试验之前的许多其他特性. Once preclinical testing is complete, 新药研究(IND)申请被提交给监管机构, such as the U.S. Food and Drug Administration, 因此,监管机构可以评估候选产品的安全性,并确保临床试验参与者不会遭受不合理的风险.
Clinical trials are conducted in four phases:
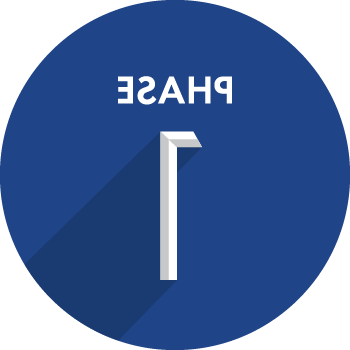
A study drug is evaluated in people, often in volunteers without the condition, 首次评估其安全性和管理候选产品的最佳实践.

评估候选产品以确定安全剂量或剂量范围, to further evaluate its safety, 然后在有兴趣条件的参与者中开始测试以确定它是否有预期的或预测的效果.

The product candidate is evaluated, often in larger trials of longer duration, to confirm its effectiveness and to further evaluate safety. 第三阶段试验通常将研究药物与常用治疗(如果有的话)或安慰剂治疗进行比较, if it is scientifically and ethically appropriate to do so.

上市后研究是在监管机构批准后进行的. 这些研究旨在收集额外的信息,包括药物的风险, benefits and optimal use in a broader patient population, often over extended periods of time. For example, 第四阶段的研究可以是对患有某种疾病的患者进行登记,以收集医疗信息并更好地了解结果, both in patients receiving the medicine and those who are not.
When Phase 1–3 clinical trials are complete, a regulatory application is submitted to regulatory agencies. 该申请包含从临床前研究和已进行的临床试验中收集的有关研究药物的安全性和有效性的所有数据. The application also contains information about the chemistry, toxicology, pharmacology and manufacturing processes of the product. The regulatory agency reviews the data and, if approved, 这种新药可以通过合格医生的处方向公众销售和分发.
When testing potential new therapies in clinical trials, 将尽可能广泛的个人纳入其中是至关重要的, 确保这些药物有最好的机会改善最终接受它们的各种患者的预后. At 博彩平台网址大全, 博彩平台网址大全为世界各地的遗传病患者创造开创性的药物, 博彩平台网址大全坚定地致力于在博彩平台网址大全的临床试验中招募具有代表性的人群.